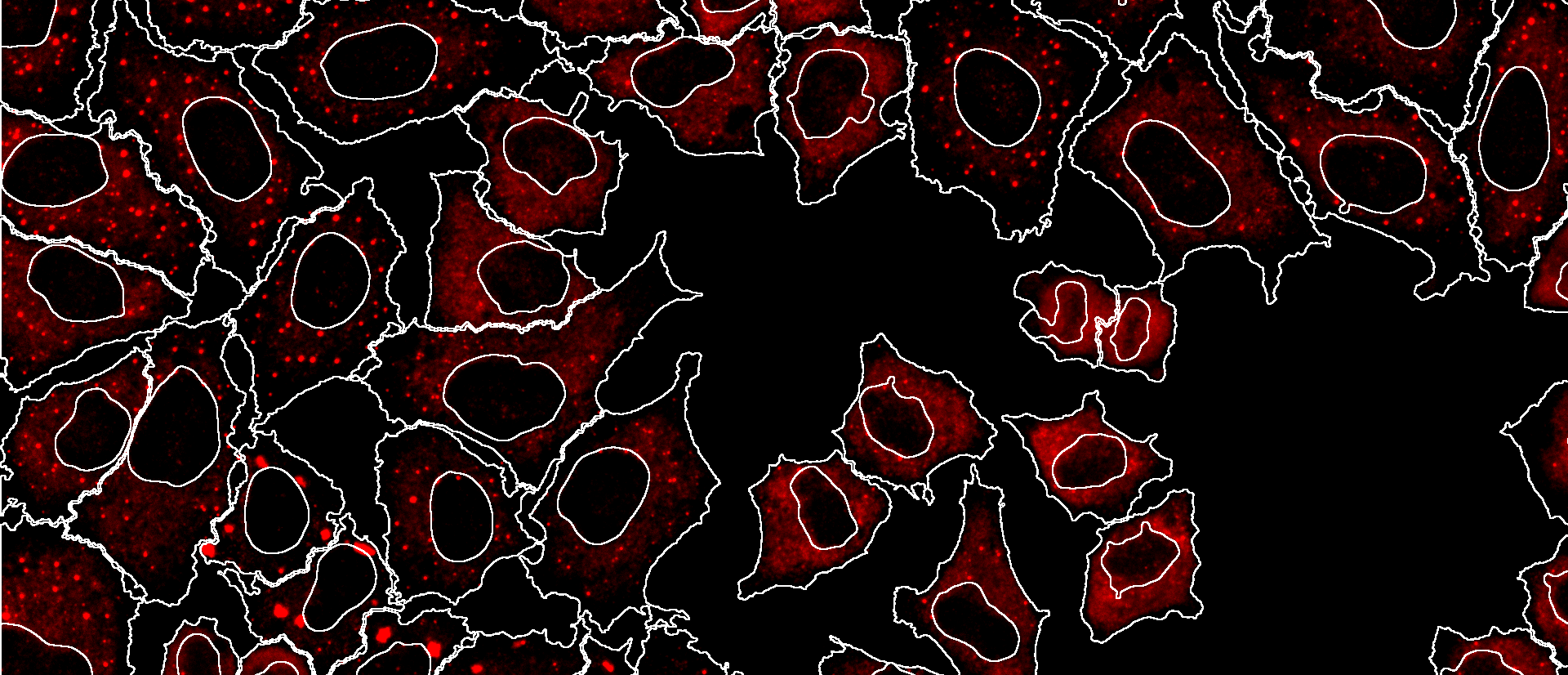
Phase separation
The cyto- and nucleoplasm of mammalian cells are highly crowded inhomogeneous mixtures of biomolecules, which have the physicochemical propensity to display a phenomenon known as complex coacervation, resulting in liquid-liquid phase separation. Phase separation is a classical emergent, self-organising property of molecules. Inside cells, this is actively harnessed to achieve a variety of poorly understood scale-crossing effects, such as intracellular compartmentalisation without membranes, the local modulation of biochemical reactions, and buffering. We are particularly interested in delineating these scale-crossing effects, how these are regulated, and how intracellular phase separation is pushed out of equilibrium and across critical boundaries during various cell physiological processes.
Time-lapse movie showing fusion of phase-separated liquid-like droplets formed by a secretory pathway protein in HeLa cells. Movie recorded by Arpan Rai (2019).
Dual-specificity kinase DYRK3
Our lab was the first to discover that a specific kinase, the dual-specificity kinase DYRK3, acts as a regulator of intracellular phase separation by controlling the condensation threshold of its substrates. This showed that phase separation is actively regulated in cells, and that this physicochemical phenomenon is used to control signal transduction, namely by coupling the reactivation of protein translation to the dissolution of stress granules. Since then, we have found that DYRK3 also plays a crucial role during mitosis to mediate the dissolution of both cytoplasmic and nuclear membraneless organelles upon nuclear envelope breakdown, and to prevent their aberrant co-condensation in mitotic cells, which is important for correct mitotic spindle assembly and kinetochore formation.
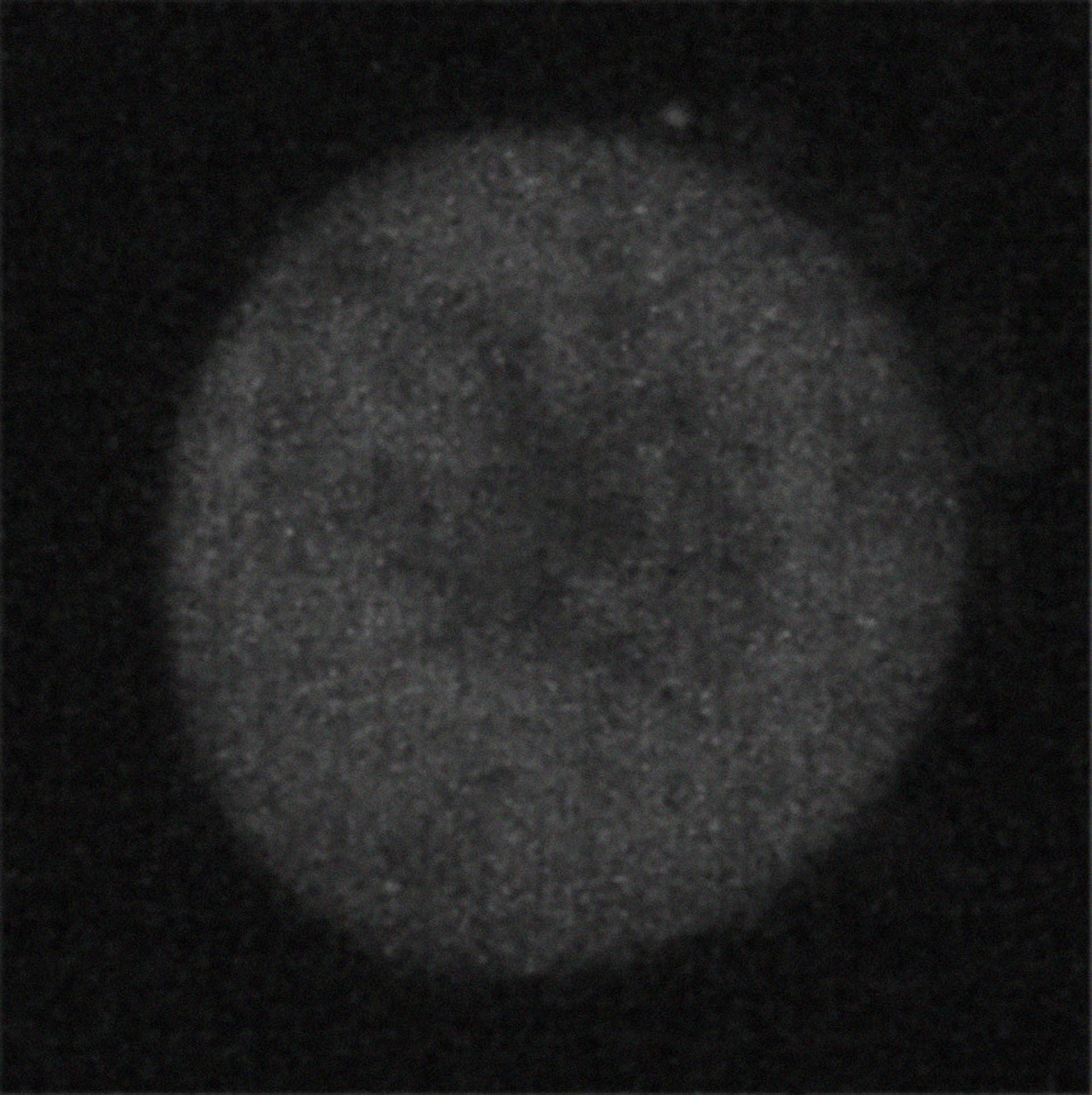
SRRM2 in mitotic cells before DYRK3 inhibition
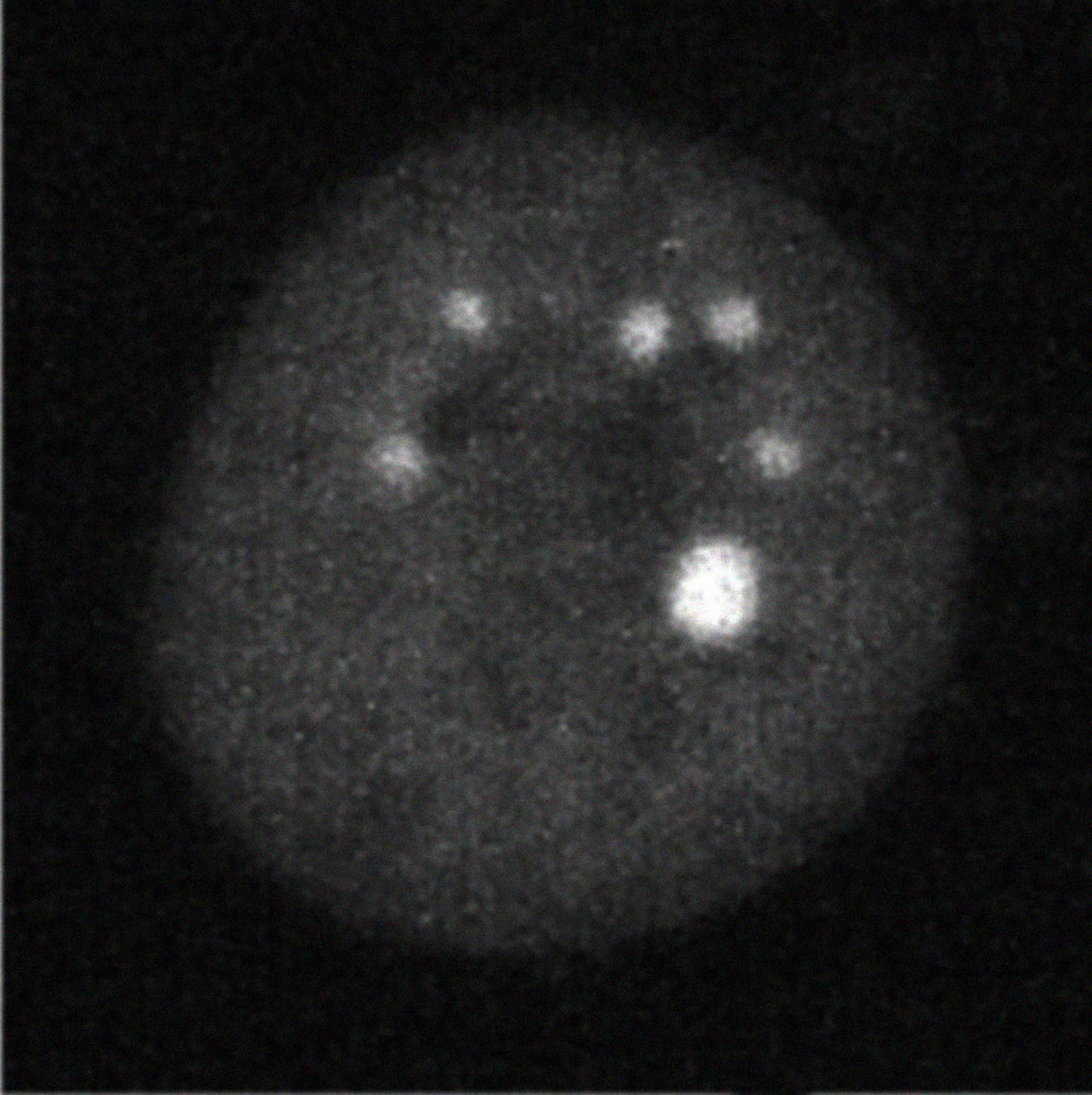
... 18 min after DYRK3 inhibition
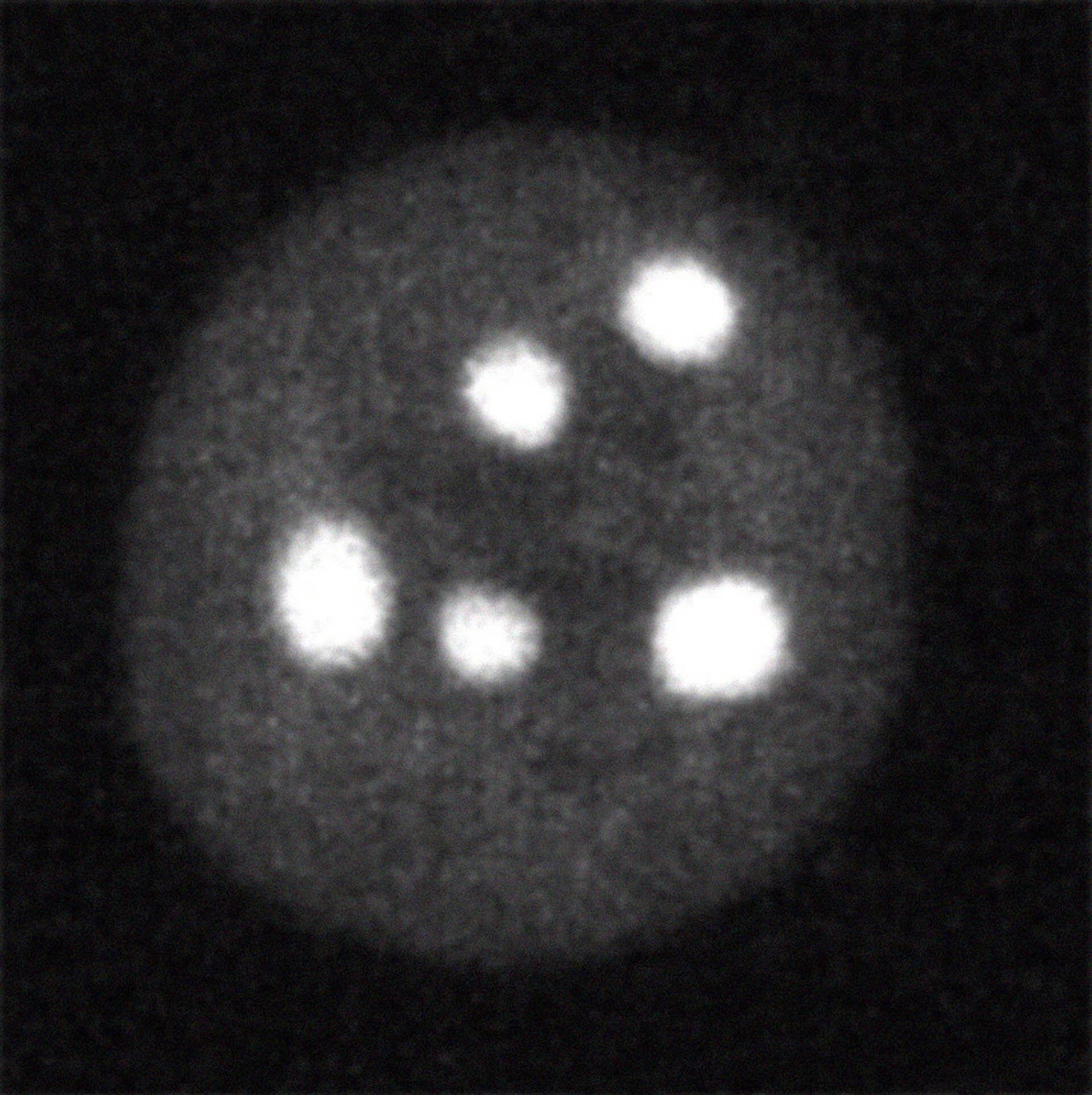
and 27 min after DYRK3 inhibition
Regulated phase separation in cell physiology
Currently, we are exploring other phase separation-based mechanisms controlled by dual-specificity kinases, which is bringing us to the fields of membrane trafficking, DNA replication, and chromatin. We are mapping the molecular complexity that underlies the homeostatic regulation of membraneless organelles and what their roles are in cellular physiology by employing proteomics and high-throughput image-based screens. And we are investigating the details of the cyclic mechanism by which dual-specificity kinases operate, as well as the role of phosphatases, using in vitro reconstitution and quantitative functional imaging.
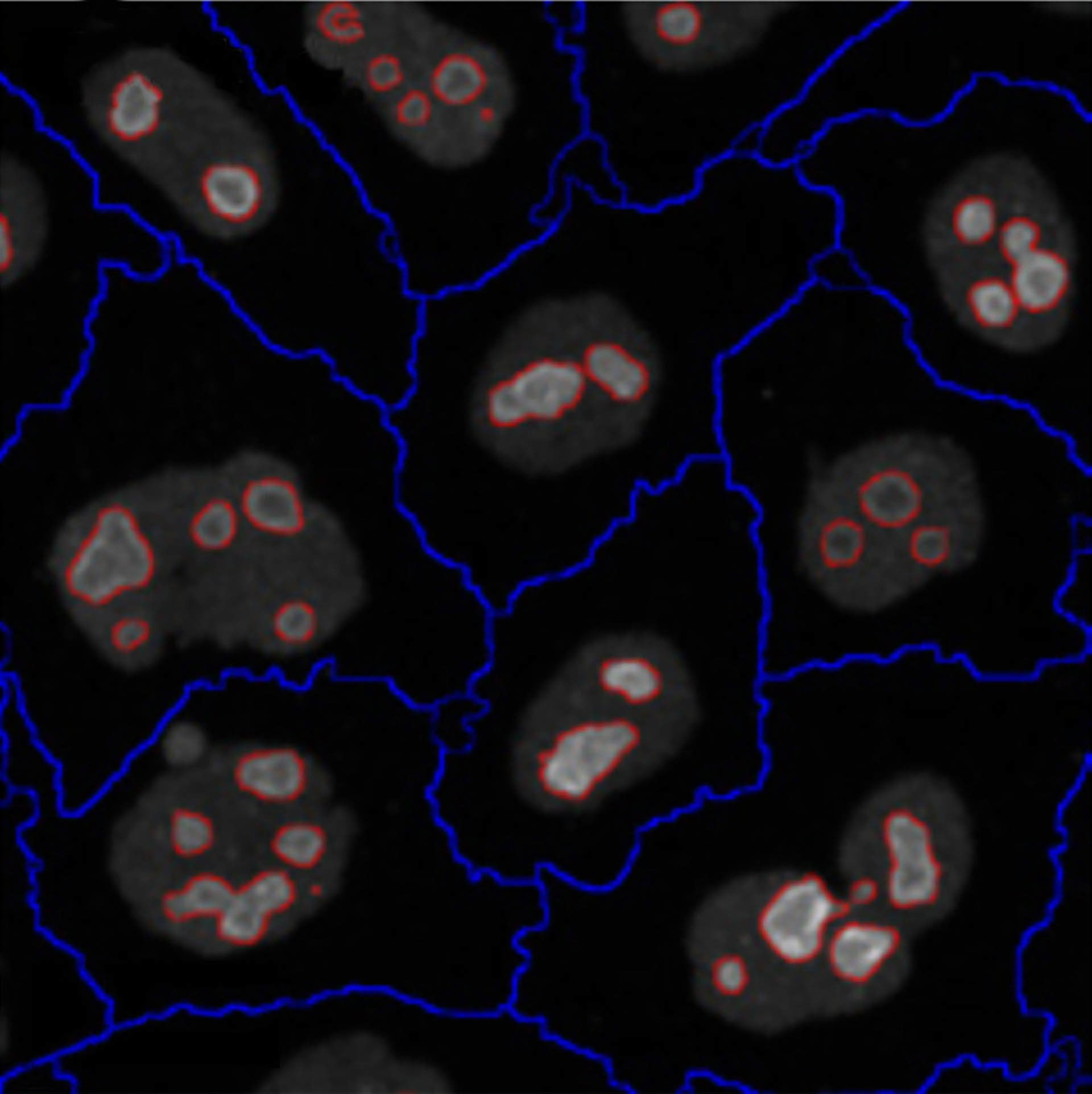
Berchtold et al. (2018). Image-based screen of nucleoli: Control
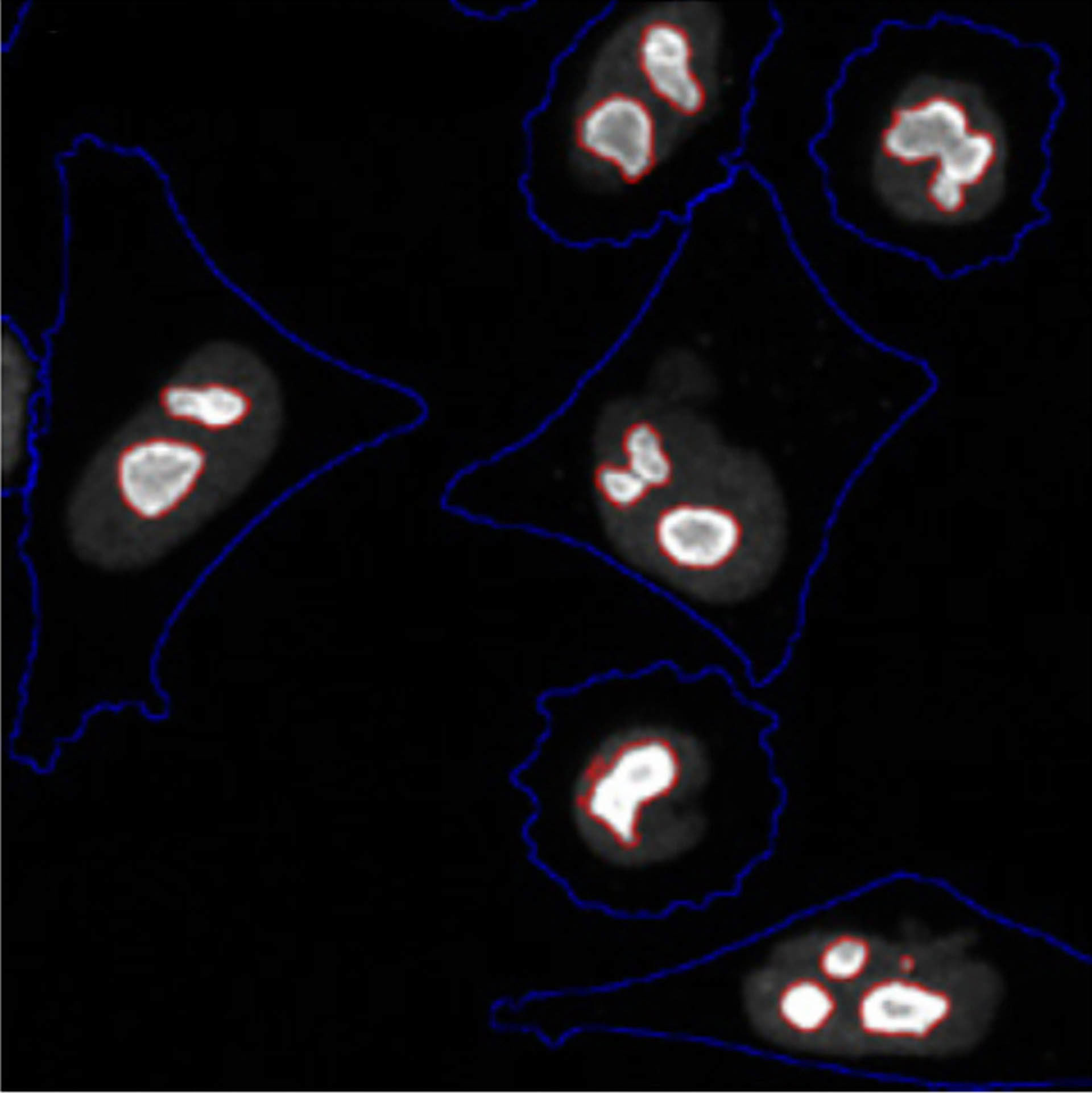
...knockdown of PSMC3
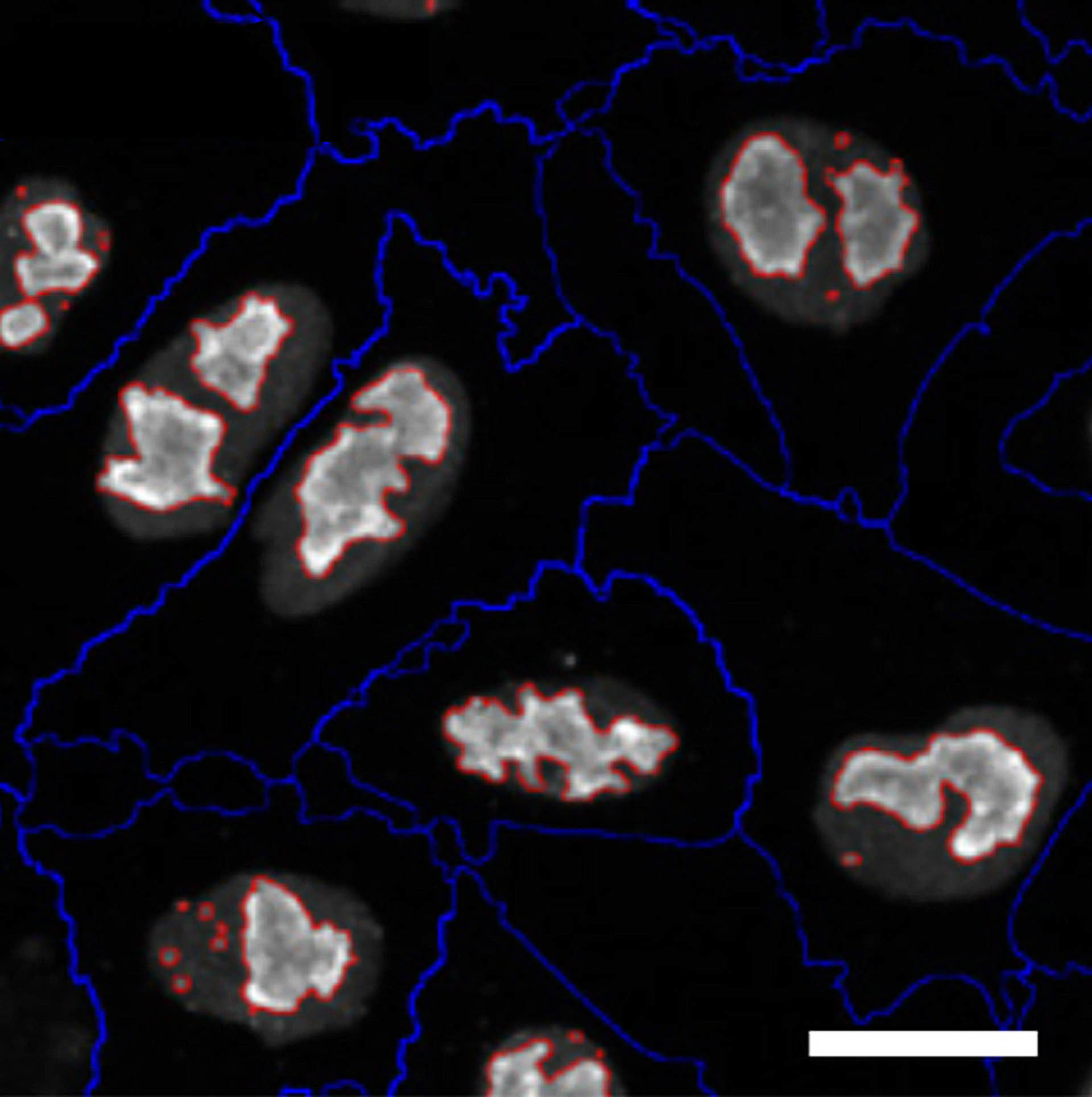
...knockdown of EDC3
Selected publications: